INFLAMATION
Definition
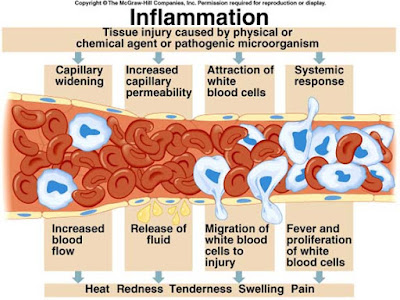
Inflammation
is defined as the local response of living mammalian tissues to injury ie
harmful Stimuli such as pathogens or irritant (Wherein injury is combated and damage
repaired). It is body defense reaction in order to elimination the spread of
injurious agent as well as to remove the necrosed cells.
Clinical Feature
·
Rubor
(Redness)
·
Tumor
·
Color
(Heat)
·
Dolor
(Pain)
·
Functiolaesa
(Loss of Funtion)
Types of Inflammation
Depending
upon the defense capacity of host & duration of response, inflammation can
be classified as:-
1.
Acute
Inflammation.
2.
Chronic
Inflammation.
Acute
Inflammation
It
represent the earliest feature of inflammation response to injury & its
duration of response is short, characterized by exudation of fluid & plasma
proteins (edema) and emigration of Leukocytes & neutrophils.
Chronic Inflammation
Inflammation
is generally described as chronic wnen it last 6 month or longer.
Sequence
of event leading to inflamation
- 1. Damage to cells, blood vessels and blood components.
- 2. Release of vasoactive substances (histamine, serotonin, bradykinin, thromboxanes, prostaglandins)
- 3. Vasodilatation of local capillaries.
- 4. Increased permeability of capillaries.
- 5. Exudation of fluid into the interstitial spaces.
- 6. Fibrin formation through fibrinogen.
- 7. Formation of edema/haematoma (causing Tumor or swelling and eventually loss of function)
- 8. Fibrinolysis leading to improved circulating.
- 9. Proliferation and repair processes.
The
changes in acute inflammation can be described under following two heading:
I)
Vascular event and II) Cellular event
I)
Vascular event:
Vasodilation and increased permeability
As defined, acute inflammation is an
immunovascular response to an inflammatory stimulus. This means acute
inflammation can be broader divided into a vascular phase that occurs first,
followed by a cellular phase involving immune cells (more specifically myeloid granulocytes in the acute setting). The vascular
component of acute inflammation involves the movement of plasma fluid, containing important proteins such as fibrin and immunoglobulins (antibodies), into inflamed tissue.
Upon contact with PAMPs, tissue macrophages and mastocytes release vasoactive amines such as histamine and serotonin, as well as eicosanoids such as prostaglandin E2 and leukotriene
B4 to remodel the local
vasculature. Macrophages and endothelial cells release nitric oxide. These mediators
vasodilate and permeabilize the blood
vessels, which results in the net distribution of blood plasma from the vessel into the tissue space.
The increased collection of fluid into the tissue causes it to swell (edema).
This exuded tissue fluid contain various antimicrobial mediators from the
plasma such as complement, lysozyme, antibodies, which can immediately deal
damage to microbes, and opsonise the microbes in preparation for the cellular
phase. If the inflammatory stimulus is a lacerating wound, exuded platelets, coagulants, plasmin and kinins can clot the wounded area and provide haemostasis in the first instance. These clotting
mediators also provide a structural staging framework at the inflammatory
tissue site in the form of a fibrin lattice - as would construction scaffolding at a construction site -
for the purpose of aiding phagocytic debridement and wound repair later on. Some of the exuded tissue
fluid is also funneled by lymphatics to the regional lymph nodes, flushing
bacteria along to start the recognition and attack phase of the adaptive immune system.
Acute inflammation is characterized
by marked vascular changes, including vasodilation,
increased permeability and increased blood flow, which are induced by the
actions of various inflammatory mediators. Vasodilation occurs first at the arteriole level, progressing to the capillary level, and brings about a net increase
in the amount of blood present, causing the redness and heat of inflammation.
Increased permeability of the vessels results in the movement of plasma into the tissues, with resultant stasis due to the increase in the
concentration of the cells within blood - a condition characterized by enlarged
vessels packed with cells. Stasis allows leukocytes to marginate (move) along the endothelium, a process critical to
their recruitment into the tissues. Normal flowing blood prevents this, as the shearing force along the periphery of the vessels
moves cells in the blood into the middle of the vessel.
II)
Cellular event
The cellular component involves leukocytes, which normally reside in
blood and must move into the inflamed tissue viaextravasation to aid in inflammation. Some act as phagocytes, ingesting bacteria, viruses, and cellular
debris. Others release enzymatic granules that damage pathogenic invaders.
Leukocytes also release inflammatory mediators that develop and maintain the
inflammatory response. In general, acute inflammation is mediated by granulocytes, whereas chronic
inflammation is mediated by mononuclear cells such as monocytes and lymphocytes.
a)Leukocyte
extravasation
Various leukocytes, particularly neutrophils,
are critically involved in the initiation and maintenance of inflammation.
These cells must be able to move to the site of injury from their usual
location in the blood, therefore mechanisms exist to recruit and direct
leukocytes to the appropriate place. The process of leukocyte movement from the
blood to the tissues through the blood vessels is known as extravasation, and can be
broadly divided up into a number of steps:
1) Leukocyte
margination and endothelial adhesion: The white blood cells within the vessels which are
generally centrally located move peripherally towards the walls of the vessels. Activated macrophages in the tissue
release cytokines such as IL-1 and TNFα, which bind to their respective G protein-coupled receptors on the endothelial wall. Signal transduction induces the immediate expression of P-selectin on endothelial cell surfaces. This
receptor binds weakly to carbohydrate ligands on the surface of leukocytes and
causes them to "roll" along the endothelial surface as bonds are made
and broken. Cytokines from injured cells induce the expression of E-selectin on endothelial cells, which functions
similarly to P-selectin. Cytokines also induce the expression of integrin ligands such as ICAM-1 and VCAM-1 on endothelial cells, which mediate
the adhesion and further slow leukocytes down. These weakly bound leukocytes
are free to detach if not activated by chemokines produced in injured tissue.
Activation increases the affinity of bound integrin receptors for ICAM-1 and
VCAM-1 on the endothelial cell surface, firmly binding the leukocytes to the endothelium.
2) Migration across
the endothelium, known as transmigration, via the process of diapedesis: Chemokine gradients stimulate the adhered leukocytes to
move between adjacent endothelial cells. The endothelial cells retract and the
leukocytes pass through the basement membrane into the surrounding tissue using
adhesion molecules such as ICAM-1.
3)Movement of
leukocytes within the tissue via chemotaxis: Leukocytes reaching the tissue interstitium bind toextracellular
matrix proteins via expressed
integrins and CD44 to prevent them from leaving the site.
A variety of molecules behave as chemoattractants,
for example, C3a or C5, and cause the leukocytes to move along a chemotactic
gradient towards the source of inflammation.
b)Phagocytosis
Extravasated neutrophils in the
cellular phase come into contact with microbes at the inflamed tissue. Phagocytes express cell-surface endocytic pattern recognition receptors (PRRs) that have affinity and efficacy
against non-specific microbe-associated
molecular patterns (PAMPs). Most
PAMPs that bind to endocytic PRRs and initiate phagocytosis are cell wall components, including
complex carbohydrates such as mannans and β-glucans, lipopolysaccharides (LPS),peptidoglycans, and surface
proteins. Endocytic PRRs on phagocytes reflect these molecular patterns, with C-type lectin receptors binding to
mannans and β-glucans, and scavenger
receptors binding to LPS.
Upon endocytic PRR binding, actin-myosin cytoskeletal rearrangement adjacent to the plasma
membrane occurs in a way that endocytoses the plasma membrane containing the
PRR-PAMP complex, and the microbe. Phosphatidylinositol and Vps34-Vps15-Beclin1 signalling pathways have been
implicated to traffic the endocytosed phagosome to intracellular lysosomes,
where fusion of the phagosome and the lysosome produces a phagolysosome. The reactive oxygen species,superoxides and hypochlorite bleach within the phagolysosomes then
kill microbes inside the phagocyte.
Phagocytic efficacy can be enhanced
by opsonization. Plasma derived
complement C3b and antibodies that exude into the
inflamed tissue during the vascular phase bind to and coat the microbial
antigens. As well as endocytic PRRs, phagocytes also express opsonin receptors Fc receptor and complement
receptor 1 (CR1), which bind to
antibodies and C3b, respectively. The co-stimulation of endocytic PRR and
opsonin receptor increases the efficacy of the phagocytic process, enhancing
the lysosomal elimination of the infective agent.